


Comparison of Intramuscular Pentazocine and Intramuscular Diclofenac for Pain Relief During Manual Vacuum Aspiration at Federal Teaching Hospital, Katsina - A Randomized Control Trial.
Abe Abidemi Job1, Tukur Jamilu1, Aisha Abdurrahman1, Nura Abdulkarim1.
1Department of Obstetrics and Gynaecology, Federal Teaching Hospital, Katsina.
ABSTRACT
Background: Pentazocine is a commonly used drug for pain relief during Manual vacuum aspiration (MVA). It is associated with some side effects, thus the need to find an alternative with similar effectiveness and less side effects. Objective: We compared the effectiveness of intramuscular Pentazocine with intramuscular Diclofenac as pain relieving drugs during MVA. Research Method: This was a randomized double blind controlled trial conducted at Federal Teaching Hospital (FTH), Katsina. Each patient received either Pentazocine or Diclofenac before the MVA. Questionnaires were filled to record the pain score (Visual Analogue Scale VAS), maternal satisfaction (Likert scale) and side effects of the drugs in both groups. Statistical analysis was done using computer software SPSS version 22.0. Results: The median difference in VAS (Inter Quarter Range IQR) for the Pentazocine group was 25.5(10.00-40.00) while that of the Diclofenac group was 30.0(6.0-37.75), (U=1217.00, p=0.820); this difference was not statistically significant. Majority of both groups were very satisfied with the drugs; Pentazocine group were 44.0% Diclofenac group were 52.0%. (X2=3.36, p=0.50), the maternal satisfaction showed no statistically significant difference. There were statistically significant differences when nausea (X2=5.26, p=0.020), drowsiness (X2=25.09, p=<0.001) and sedation (X2=4.45, p=0.035) were compared between the two groups with those in the Pentazocine arm experiencing more of these side effects. Conclusion: The efficacy of intramuscular Pentazocine was comparable to that of intramuscular Diclofenac for pain relief during MVA, but intramuscular Pentazocine was associated with significant side effects. These findings showed that Diclofenac is not only as effective as Pentazocine but also safer as pain relieving drug for patients during MVA at FTH, Katsina.
Keywords: Manual Vacuum Aspiration, pain management, Pentazocine, Diclofenac.
Corresponding Author:
Dr. Abe Abidemi
Department Of Obstetrics and Gynaecology, Federal Teaching Hospital, Katsina
Phone numbers: +2347038130806
drbidemi@yahoo.com
Over the past few decades, manual vacuum aspiration has emerged as an effective and safe procedure for surgical management of miscarriage. One of the problems of manual vacuum aspiration is pain which is caused by anxiety, cervical dilatation, manipulation of the cervix, uterine suction and evacuation1. It was documented that 97% of women report moderate to severe intensity pain during and immediately following a manual vacuum aspiration making pain relief necessary2. A complex set of pathway transmits pain messages from the periphery to the central nervous system, where control occurs in the higher centres3.
Manual vacuum aspiration is a common procedure done as up to 88% of women with miscarriage undergo surgical evacuation4. One of the most important aspects in treating incomplete abortion is the sufficient management of pain during the process of manual vacuum aspiration with minimal risk to the patient’s health and maximum satisfaction. Pain is one of the main postoperative adverse outcomes causing distress to patients5. It leads to patient’s discomfort, decreased level of satisfaction and higher level of health care cost6,7.
About 10-20% of pregnancies end up as miscarriage8 and most of these miscarriages are treated with manual vacuum aspirationmaking the procedure the commonest in gynecological practice9. There are different pharmacological options of pain relief during MVA. Pentazocine and Diclofenac are two of the options.
Pentazocine is effective in reducing moderate to severe pain and it is the commonly used drug in our centre. It causes drowsiness, nausea and vomiting thus having potentials to increase morbidity and period of hospitalization in the patient10. On the other hand, Diclofenac has been shown to have less side effects than Pentazocine (opioids) during MVA in some studies11,12.
The best method that will provide good pain relief with minimal or no side effects has not been established. Several studies have shown that opioids have superior analgesic effect 13,14 while others found no difference as women still experience pain during uterine intervention irrespective of the analgesic technique used15,16. Furthermore, a Cochrane review noted that most of the studies on pain relief during MVA are at risk of bias due to lack of blinding and lack of allocation concealment17. Several studies, including a recent meta-analysis that reviewed the methods of pain relief during MVA, have concluded that there is insufficient evidence to recommend one method of pain relief over another and recommended that more studies should be done 18,19,20, 21.
This study was carried out to compare the effectiveness of Pentazocine with Diclofenac in reducing pain during MVA at Federal Teaching Hospital, Katsina. The findings will hopefully provide evidence that would be used to improve pain management in patients during MVA in our centre. The objectives of this study were to compare pain perception, maternal satisfaction and side effects between those who had Pentazocine injection and those who had Diclofenac injection for pain relief during MVA.
METHOD
It was a double-blind randomized control trial that involved all consenting women who presented with miscarriage of less than 12weeks for MVA. The obese, the unconscious and patients with adverse reaction to either of the drugs were excluded. The study was conducted over an eight-month period from August 2020 to March 2021.
The sample size was calculated using the formula22.
N= (Zα+Zβ)2S2
d2
Zα was determined from a statistical table based on the value of the α-level of significance for this study, it was set at 0.05, therefore Zα= 1.96.
Zβ was determined from a statistical table based on the acceptable power of comparison between 2 groups. For this study power of 95% (0.95) was used. Zβ=1.64
S= pooled standard deviation
d= mean difference in pain score.
In a similar study in Kano23, standard deviation was 2.8, and mean difference in pain score was 1.5,
N= 

N= 

N= 
 =
= 
N=  = 45.16. Attrition rate is 10% of N.
= 45.16. Attrition rate is 10% of N.
Assuming the attrition rate was 5%, adding the attrition rate to N makes the final population size to be 50 participants per arm. Fifty patients were recruited in each group thus making a total of one hundred patients in both groups.
Patient Recruitment
Patients were recruited consecutively using purposeful sampling technique. All patients were reviewed to determine their eligibility for recruitment. At the point of recruitment, patients were counseled on participation. An informed consent was taken from those that met the inclusion criteria and accepted to participate in the study. The patients were divided into group A and B by simple randomization that was computer generated.
Randomization and Blinding
One hundred serial numbers were randomized into 2 groups with 50 patients in each group using simple randomization generated using SPSS software version 22.0. The groups were labelled as A and B. The group (A or B) was written in an opaque envelope and the serial number was written at the back of the envelope. The envelopes were arranged serially in a box and one envelope was drawn for each consecutive patient recruited. The patients were then assigned to the group written in the envelope after it has been opened. Patients randomized to group A received the drug labelled A while patients randomized to group B received the drug labelled B.
The two drugs (75mg Diclofenac injection and 30mg Pentazocine injection) were constituted with the same colour, quantity, volume, consistency, packaging and route of administration (intramuscular). The pharmacy department of Ahmadu Bello University Teaching Hospital, Zaria packaged the drugs for blinding. One of the drugs was labelled as A while the other was labelled as B. The study was double blind as both the research team and the patients did not know the drug being administered. The drugs were dispensed by a pharmacist who was blinded to the protocol of the study.
Data Collection
Following recruitment and randomization, the patients were prepared for MVA. The initial pain perception of the patients was assessed using VAS before commencement of the procedure. First aid box was provided to manage emergency complications. The procedure was clearly explained to the patients. Patients assigned to group A received an intramuscular injection in the gluteal muscle of the thigh from the ampoule labelled A while those in group B received an intramuscular injection in the gluteal muscle of the thigh from ampoule labelled B. Injections were given within 20-30 minutes before commencement of the procedure after which the MVA was done according to the unit protocol by the attending doctor the cadre of which was noted on the questionnaire. Within the first 10mins of MVA, questionnaire was administered where she marked her pain perception using VAS24, she rated her satisfaction level using Likert scale25 and she filled the side effects chart. Other details such as the socio demographic characteristics, obstetric history and MVA details were also recorded. The time of administration of the injection was recorded. The time of insertion of the speculum was recorded as the zero minute of the commencement of the procedure and the time of removal of the speculum was recorded as the time of completion of the procedure. Patients who requested for extra analgesia during or after the procedure were given intramuscular Paracetamol 600mg stat.
Statistical Analysis
The questionnaires were analyzed at the end of the study. The information obtained from the study and findings from the data analysis were presented in tabular forms using different variables. Normally distributed quantitative variables were summarized as means and standard deviations and compared using Student t test while those that were not normally distributed were summarized as medians and interquartile ranges (IQR) and compared using Mann-Whitney U test. Categorical variables were summarized using numbers and frequencies and compared using Chi square or Fisher’s exact test as appropriate. A p value of less than 0.05 was considered to be statistically significant.
Ethical Consideration
Ethical approval was obtained from the Health Research and Ethics Committee of FTH, Katsina. Only those patients who gave informed written consent were enrolled. The provisions of the Helsinki declaration (2013) on investigation of human subjects were adhered to.
RESULTS
The consult guideline 2010 for reporting parallel group RCT was used. Below is the flow diagram of the process from enrolment to analysis. Table I shows the socio demographic characteristic of the patients. The mean age of the patients in group A was 28.06±6.89 years while that of group B was 28.50±6.49 years, the difference was not statistically significant (t=-0.33, p=0.743). Generally, there were no statistically significant differences in the socio demographic characteristics of the patients in the two groups.
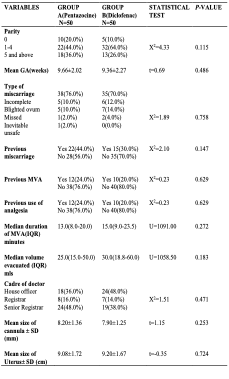
Table II shows the obstetric characteristics of the patients. In group A, 44.0% of the patients were para 1 to para 4 while 36.0% of the patients were grand multiparas. In group B, 64.0% of the patients were para 1 to para 4 while 26.0% of the patients were grand multiparas. There was no statistically significant difference in the distribution of the patients

Fig. 1. The consult guideline 2010 for reporting parallel group RCT was used. Below is the flow diagram of the process from enrolment to analysis.
Table I: Socio Demographic Characteristics of Patients

*Banker, Hairdresser, Tailor
by parity (X2=4.33, p=0.115). The mean gestational age for the patients in group A was 9.66±2.02 weeks and 9.36±2.27 for the patient in group B. The difference was not statistically significant (t=0.69, p=0.486). Generally, there was no statistically significant difference in the distribution of patients by their obstetrics characteristics.
Table II: Obstetrics Characteristics of Patients

Table III shows the pain perception scores of the patients. The median VAS scores before the administration of analgesia were 40.0(IQR 28.75-60.00) mm in group A and 43.0 (IQR 29.5-55.25) mm in group B. The difference was not statistically significant (Mann-Whitney U test=1208.00, p=0.771). The median VAS scores after the administration of analgesia were 10.0(IQR 4.75-27.75) mm in group A and 15.0(IQR 10.00-30.50) mm in group B. The difference was also not statistically significant (Mann-Whitney U test=1039.00, p=0.143). The median difference in VAS scores in group A was 25.5(10.00-40.00) while the median difference in VAS scores in group B was 30.0(6.00-37.75). Also, there was no statistically significant difference between the two groups. (Mann-Whitney U=1217, p=0.820).
Table III: Pain Perception

VAS Visual Analogue Scale; IQR Inter Quarter Range
Table IV: Maternal Satisfaction

Table IV showed the maternal satisfaction between the two groups. Most of the patients in the two groups were very satisfied with pain relief provided by the analgesia (44.0% in group A, 52.0% in group B). The difference was not statistically significant (X2=3.36, p=0.500).
Table V shows the side effects of the drugs. Nausea occurred in 28.0% of the patients in group A and 10.0% of the patients in group B. The difference was statistically significant, and the incidence of nausea was significantly higher among patients in group A (X2=5.26, p= 0.020). Vomiting occurred in 12.0% of patients in group A and 6.0% of patients group B. The difference was not statistically significant (X2=1.09, p=0.295). In group A, 78.0% of patients felt drowsy with the administration of the drug while 28.0% of patients in group B felt drowsy with the administration of the drug. The difference was statistically significant, and the incidence of
Table V: Side Effects of the Drugs
*Statistically significant
drowsiness was significantly higher in group A (X2=25.09, p=<0.001). Only 2.0% of the patients in group A had dyspnea while none in group B had dyspnea. The difference was not statistically significant (Fisher’s exact test, p=0.315). In group A 44.0% of patients had sedation after the administration of the drug while 24.0% of the patients had it in group B. The difference was statistically significant, and the incidence of sedation was statistically higher in group A (X2=4.45, p=0.035). In group A 4.0% of patients had epigastric pain while 10.0% of the patients in group B had epigastric pain. The difference was not statistically significant (X2=1.38, p=0.240). The mean systolic blood pressure of patients in group A was 109.00±11.33mmHg and the mean systolic blood pressure of the patients in group B was 109.00±10.59mmHg. The difference was not statistically significant (t=0.18, p=0.860). The mean diastolic blood pressure of the patients in group A was 73.00±10.15mmHg while the mean diastolic blood pressure of the patients in blood B was 75.00±8.83mmHg. The difference was not statistically significant (t=-1.21, p=0.230).
DISCUSSION
Pain management remains a major aspect of care given to patients who undergo manual vacuum aspiration for miscarriage and therefore analysis to assess an effective method of care is essential in ensuring a good outcome after the procedure. This was a randomized controlled study conducted among patients who had manual vacuum aspiration at Federal Teaching Hospital, Katsina to compare the effectiveness of intramuscular Pentazocine with intramuscular Diclofenac as pain relieving drugs during manual vacuum aspiration. It was evident that there were no statistically significant differences between the two groups in terms of their socio demographic characteristics and obstetric characteristics.
From the outcome of this study, the difference in the pain perception following the administration of the analgesia between the two groups was not statistically significant as shown by the statistically similar median difference in VAS score (p=0.820). This means that the effectiveness of intramuscular Pentazocine is similar to that of intramuscular Diclofenac in providing pain relief during MVA. This is contrary to the findings in studies conducted by Lowenstein et al26 and Khazin et al13.
Lowenstein et al found that there was a significant difference in pain levels between the two groups of medication with rectal indomethacin (NSAID) having a lower VAS score when compared with rectal tramadol (opioid) during manual vacuum aspiration (p<0.0001). Their efficacy was assessed by VAS score and by the number of women who requested for rescue analgesia; fewer women in the NSAID group requested for rescue analgesia. In that study, the researchers did not take account of the weight of the patients and the allocation of patients into groups was not randomized. These two factors could have affected their results. The study conducted by Khazin et al found that tramadol (opioid) showed greater analgesic efficacy than indomethacin (NSAID) as assessed by VAS (p=0.005).
The analgesic effects of tramadol appear to be produced in a multimodal mechanism involving the opioid system, the noradrenergic system and the serotonergic system; these could probably explain the reason for this conflicting result. In that study, there was no consideration on how far into the rectum the suppositories were inserted in each patient; this could have led to differences in the bioavailability of the drugs ultimately affecting the pain perception of the patients. The finding in this study was similar to that of Romero who found that there was no statistically significant difference in immediate postoperative pain between women who received tramadol and those who received ibuprofen during manual vacuum aspiration(p>0.05)27. This is probably because the two drugs have the same onset and duration of action (onset of action 30minutes and duration of action 6 hours).
The maternal satisfaction with pain relief during manual vacuum aspiration as assessed using Likert scale in the two groups showed no statistically significant difference; both groups were very satisfied with the drugs. This was similar with findings reported by other studies23,28,29. In one of the studies, the maternal satisfaction with pain control after the administration of Hydrocodone (opioid) was comparable with that of placebo during manual vacuum aspiration. That study was a randomized controlled trial that was conducted for nine months to assess the efficacy and maternal satisfaction of Hydrocodone compared with placebo in relieving painrelated to first trimester surgical abortion. Mean satisfaction with pain control was 67.3 and 74.8 for the Hydrocodone and the placebo group respectively (p=0.10)28. The blinding method was such that tablet Hydrocodone was compared with a methylcellulose powder that was the placebo. There was no statistically significant difference between these groups of medication. The finding in this study was also similar to the one at AKTH, Kano where patient’s satisfaction using combined Diclofenac and Pentazocine was compared with Pentazocine alone. Patient satisfaction was better in the combined group (82.2%) compared to the Pentazocine group (66.6%).
Women who received only Pentazocine had higher dissatisfaction (13.4%) compared with women in the other group (6.7%). That study showed no statistically significant difference between the two groups as participants of both groups were satisfied with the two types of treatment given (p=0.23)23. This might be connected with the age-long preference of injection (injections were used in that study) not necessarily because of the pain relief experience by the participants.
Majority of the participants in the Pentazocine group had side effects to the drug with 78.0% of patients experiencing drowsiness (p=<0.001) and 28.0% of patients experiencing nausea (p=0.020). This was similar to a randomized control trial conducted by Ephraim at JUTH30, comparing paracervical block with intramuscular Pentazocine for pain relief during MVA. In his study also, majority of the participants who had intramuscular Pentazocine had side effects to the drug with 78.0% of them experiencing dizziness (P=0.0005) and 38% of them experiencing nausea (p=0.003). This might be due to the systemic distribution of the drug with a central nervous system action resulting in a multisystem involvement. Also, in a study in AKTH Kano23, the side effects of Pentazocine that were commonly reported were dizziness, nausea, and vomiting and there were significantly more side effects in the group of patients who had Pentazocine, which was a similar finding in this study.
In this study, few patients in the Diclofenac arm had side effects. Nausea was reported in only 10% of patients, vomiting in 6%, drowsiness in 28%, sedation in 24%, epigastric pain in 10% and no patient had dyspnea. These findings were similar to a study conducted by Lopez15, a randomized comparison of different methods of analgesia in abortion using manual vacuum aspiration. It was concluded in that study that adverse effects were fewer among patients who had Diclofenac as against Meperidine during MVA. This was probably because of the inhibitory action of the synthesis of PGE2 and thromboxane A2 by the NSAID. There were statistically significant differences when nausea (p=0.020), drowsiness (p=<0.001), and sedation (p=0.035) were compared between the two groups with those in the pentazocine arm experiencing more of these side effects. There was no statistically significant difference in vomiting (p=0.295), dyspnea (p=0.315) and epigastric pain (p=0.240) between the two groups. Also, in a study by Elizabeth et al28, there was a statistically significant difference when nausea was compared between the two groups of drugs; patients who received opioid (Hydrocodone) had more postoperative nausea than patients in the other arm. This was similar to the result of this study.
When the hypothesis of this study was tested, there was no statistically significant difference in the pain perception between the two groups of patients. This means that the patients who had Pentazocine had similar pain perception with those who had Diclofenac. We failed to reject the null hypothesis.
CONCLUSION
In conclusion, this study showed that the efficacy of intramuscular Pentazocine was comparable to that of intramuscular Diclofenac for pain relief during MVA. Also, the maternal satisfaction with pain relief during MVA was similar when intramuscular Pentazocine was compared with intramuscular Diclofenac. However, intramuscular Pentazocine was associated with significantly more adverse effects. These findings showed that intramuscular Diclofenac is not only as effective as intramuscular Pentazocine but also safer as pain relieving drug for patients during MVA.
Recommendations
Based on the findings from this study, it is recommended that:
- Intramuscular Diclofenac can be used as a safer and equally effective alternative to intramuscular Pentazocine for pain relief during MVA.
- Women who present with miscarriage should be counseled on options on pain relief including intramuscular Diclofenac, its advantages, its side effects profile and its effectiveness to other forms of analgesia.
- A multicenter randomized triple blind study could be done to further strengthen the findings from this study.
Strengths of the Study
- The strength of the study was the fact that it was a double-blind trial, and this decreased its bias as earlier study lacked blinding17.
- The tool for measuring pain perception was visual analogue scale which has been validated and is mostly used in research and in clinical practice31.
Limitations of the Study
- Pain perception is highly subjective and differs from one person to another. The effects of the drugs could have been influenced by the subjectivity of the individual respondent.
- Lengthy explanations were needed to educate the participants on how to place a mark on the visual analogue scale.
- For the Likert scale, it was likely that people’s answers would be influenced by previous questions and frequently people avoided choosing the ‘extreme’ options on the scale because of the negative implications involved with extremists.
- Full blinding might be difficult because drowsiness, which is a common side effect of Pentazocine, might have given away the patient’s assigned group to the doctors that were blinded.
Source of Fund: Self
Conflict of Interest: Nil
REFERENCES
- Mona S. Manual vacuum aspiration: an outpatient alternative for surgical management of miscarriage. Obstet Gynaecol. 2015; 17(3):157-161.
- Gweneth B, Nicholas S, Tod F. Impact of paracervical block on postabortion pain in patients undergoing abortion under general anaesthesia. Contraception. 2009; 80(6): 578-582.
- Charlotte E. The anatomy and physiology of pain. Surgery (Oxf). 2016; 34(2): 55-59.
- Millingos DS, Mathur M, Smith NC, Ashok PW. Manual vacuum aspiration: a safe alternative for the surgical management of early pregnancy loss. BJOG. 2009; 116(9): 1268-1271.
- Adeniji AO, Atanda OO. Randomized comparison of effectiveness of unimodal opioid analgesia with multimodal analgesia in post-caesarean section pain management. J Pain Res. 2013; 6: 419-424.
- Jin F, Chung F. Multimodal analgesia for postoperative pain control. J Clin Anesth. 2001; 13(7): 524-539.
- Bamigboye AA, Hofmeyr GJ. Caeserean section wound infiltration with local anaesthetic for post operative pain relief- any benefit? S Afri Med J. 2010; 100(5): 313-319.
- Abiodun SA, Adegboyega AF, Ishaq FA, Kikelomo TA. Spontaneous abortions (miscarriage): Analysis of cases at a tertiary centre in North Centre Nigeria. J Med Trop. 2015; 17(1): 22-26.
- Drakhshan N, Nadia S, Uzma S, Naheed J. Comparison of Manual Vacuum Aspiration with Electrical Vacuum Aspiration in management of first trimester miscarriages. PJMHS. 2016; 10(2): 503-506.
- Tade AO, Salami BA, Ayoade AB. Pentazocine Pain Relief in Adult Patient with acute abdominal pain: A prospective Randomized Clinical Trial. East Cent. Afri J Surg. 2009; 14(2): 44-49.
- Arfa T, Shumaila H, Naveera S. Comparison of rectal diclofenac sodium versus intramuscular tramadol for pain relief in manual vacuum aspiration. Pak J Physiol. 2018; 14(4): 28-31.
- Sahil S, Mane M, Paranjap J. Comparison of diclofenac suppository with tramadol suppository for postoperative analgesia in abdominal hysterectomy patient. Int Med J. 2017; 4(5): 606-609.
- Khazin V, Weitzman S, Rozenzvit-podles E, Ezri T, Debby A, Golan A, et al. Postoperative analgesia with tramadol and indomethacin for diagnostic curettage and early termination of pregnancy. Int J Obstetric Anesth.2011; 20(3): 236-239.
- Rodriguez MJ, De la Torre MR, Perez-Iraola P, Fernandez-Cuervo C, Benitez P, Navarro A, et al. Comparative study of tramadol versus NSAIDs as intravenous infusion for managing postoperative pain. Curr Ther Res Clin Exp. 1993; 54(4): 375-383.
- Lopez JC, Vigil-De G, Vega JC, Ruiz E, and Vergara V. A randomized comparison of different methods of analgesia in abortion using manual vacuum aspiration. Int J Gynaecol Obstet. 2007; 99(2): 91-94.
- Smith L, Carroll D, Edwards J, Moore R, McQuay H.
Single dose ketorolac and pethidine in acute postoperative pain: systematic review with meta-analysis. Br J Anaesth 2000; 84: 48-58.
- Tangsinwatthana T, Sankomkamhang US, Lumbiganon P, Laopaiboon M. Paracervical local anaesthesia for cervical dilatation and uterine intervention. Cochrane Database Syst Rev.2013; 9; CD005056.
- Miriam EW, Abbey JH, Adrianne R, Racek M, Collen KS. Pain control options for first trimester surgical abortion: a review. Proc Obstet Gynecol. 2014; 4(2). Article 2: 1-6.
- Jackson E, Kapp N. Pain control in first-trimester and second trimester surgical termination of pregnancy: a systematic review. Contraception. 2011; 83(2):116-126.
- Solo J. Easing the pain. Pain management in the treatment of incomplete abortion. Reprod Health Matters. 2000; 8 (15): 45-51
- Rebecca HA, Rameet S. Society of Family planning clinical guidelines pain control in surgical abortion part 1- local anesthesia and minimal sedation. Contraception.2018; 97(6): 471-477.
- Zhong. B. How to calculate sample size in randomized controlled trial? J Thorac Dis. 2009; 1(1): 51-54.
- Natalia A, Galadanci H, Ibrahim SA, Mohammad Z. Comparison of effectiveness of pain management during manual vacuum aspiration using single-agent analgesic and combination. A Randomized Double-Blind Controlled Trial. Open J Obstet Gynecol. 2015, 5: 244-250.
- Penton Media. Pain measuring editor’s notes (2009). Available at www.analgesia/measuringpain.mht. Last accessed on June 2012.
- Mocumbi S, Hogherg U, Lampa E, Sacoor C, Vala A, Bergstrom A, et al. Mothers’ satisfaction with care during facility-based childbirth: a cross-sectional survey in southern Mozambique. BMC Pregnancy Childbirth. 2019; 19(1): 303. https://doi.org/10.1186/s12884-019-2449-6.
- Lowenstein L, Granot M, Tamir A, Glik A, Deutsch M, Jakobi P et al. Efficacy of suppository analgesia in postabortion pain reduction. Contraception. 2006; 74(4): 345-348.
- Romero T, Turok D, Gilliam M. A randomized trial of tramadol versus ibuprofen as an adjunct to pain control during vacuum aspiration abortion. Contraception. 2008; 77(1): 56-59.
- Elizabeth AM, Alison BE, Regina MR, Rongwei FU, William EL, Paula HB, et al. Hydrocodone-acetaminophen for pain control in first-trimester surgical abortion: a randomised controlled trial. Obstet Gynecol 2012; 120 (5): 1060-1069.
- Rafet G, Emma H, Fiona M, Allan T. Manual vacuum aspiration (MVA) in the management of first trimester pregnancy loss. Eur J Obstet Gynecol Reprod Biol. 2004; 12: 197-200.
- Ephraim S. A randomized control trial comparing paracervical block with pentazocine for pain relief during manual vacuum aspiration. 2017. Available at https://www.dissertation.npmcn.edu.ng/index.php/FMCOG/article/view/1791. Access April.
- Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14(7): 798-804.
